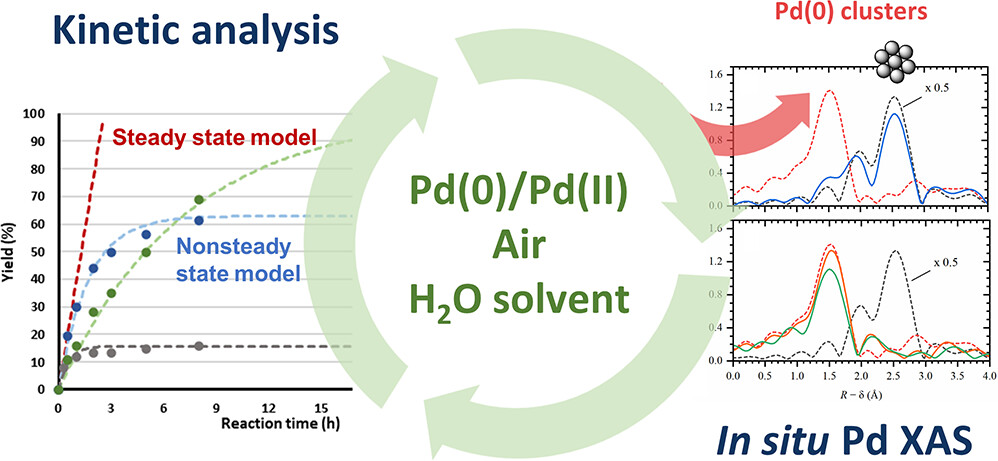
Direct activation of C-H bonds in tryptophan residues through green processes, using air as an oxidant, and lowering the amounts of noble-metal catalyst, is crucial for the sustainable and eco-friendly synthesis of multiple fine chemicals with relevant applications. Optimization of the catalytic process requires a deep understanding of the structure of active catalytic sites and their deactivation mechanisms, that were accesses in this work by non-steady state kinetic analysis and in situ X-ray absorption spectroscopy (XAS).
The procedure developed by Prof. Dirk De Vos group at KU Leuven (Belgium) veers away from conventional methods that rely on harsh conditions and non-native coupling partners. Instead, it taps into molecular oxygen, abundantly present in the air, to enable the use of easily accessible boronic acid coupling partners under mild, water-based conditions. By doing so, it not only reduces the demand for scarce resources and harsh chemicals but also minimizes waste generation. This shift towards greener chemistry aligns with the principles of the circular economy by promoting the efficient use of resources and reducing environmental impact.
In situ XAS experiments, provided by ReMade@ARI at the SuperXAS beamline of the Swiss Light Source (PSI, Switzerland) allowed to track active palladium species, present in minimal quantities in the systems, and their evolution under reaction conditions depending on the catalytic procedure.
Overall, this research illustrates a significant step towards a more sustainable and eco-friendly approach to chemical synthesis, particularly in applications within the realms of biology and medicine. The results have been recently published in Beckers et al., ACS Catalysis 2024 (DOI: 10.1021/acscatal.4c01699).